Science and Technology
- Vincere Bio
- Science and Technology
Mitophagy Removes Damaged Mitochondria to Slow Aging
Dysfunctional mitochondria cause damage to cells and are a major hallmark of aging and age-related disease.
To slow the progression of aging and age-related diseases, our molecules accelerate the removal of damaged mitochondria by increasing the natural process of mitophagy.
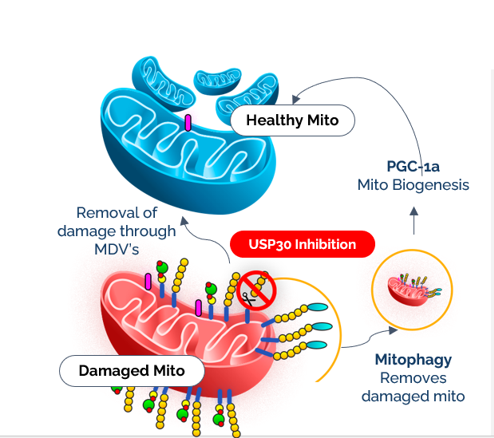
USP30 Is the Best Target to Increase Mitophagy
Cells are constantly balancing damage and repair.
When a mitochondrion is damaged, the cell tags it with ubiquitin.
That tag triggers mitophagy, the selective removal of damaged mitochondria.
There is a brake on this process, called USP30.
USP30 sits on mitochondria and removes those ubiquitin tags.
When the tags are removed, mitophagy slows down.
Damaged mitochondria accumulate.
If you inhibit USP30, you release the brake.
Mitophagy increases.
Damaged mitochondria are cleared.
Healthy ones take over.
Because mitochondrial damage drives many diseases,
small-molecule USP30 inhibitors could have broad therapeutic impact.
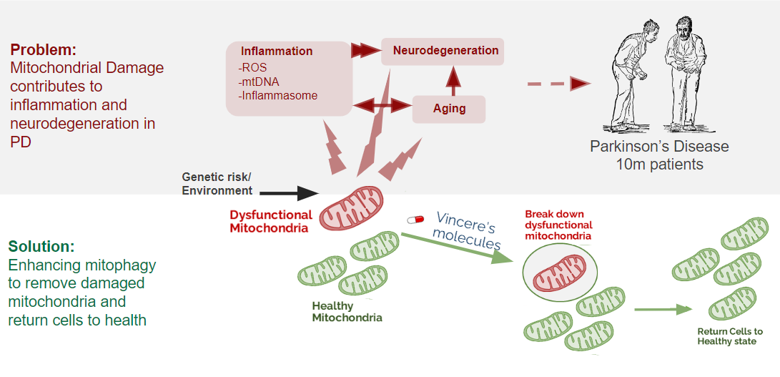
Increasing Mitophagy Has Broad Therapeutic Potential
Parkinson's Disease
Genetic mutations that disrupt mitophagy cause Parkinson’s disease and preclinical models suggest inhibiting USP30 can stop neurodegeneration, protein aggregation, and motor deficits.
Cardiorenal
Cardiomyocytes and renal tubular epithelial cells operate under sustained metabolic demand and dysfunctional mitochondria contribute to organ decline. Preclinical data suggests USP30 inhibition can stop and even reverse damage to these organs.
Aging
Mitochondrial dysfunction is a core hallmark of aging biology and increasing mitophagy leads to longer lifespan and healthspan in animals. Since aging is the dominant risk factor for most chronic diseases, improving mitochondrial maintenance is a scalable strategy to treat multiple diseases of aging.
Mitophagy enhancers validate scalable discovery platform
Hypothesis Generation
》Network Al algorithms
》Data-mining integrations
》Virtual cell simulations
Chemistry ID & Optimization
》GPU-enabled docking tools
》Automated screening workflow
Patient Selection
》Feature-selection method
》Regression Al algorithms
》Classification Al